锂消防:对于锂离子电池有效的灭火剂研究
发布时间:2024-05-06浏览次数:次
Fires involving lithium-ion cells are the result of electrolyte burning, which is a hydrocarbon/airflame. Thus, many flame suppression agents will be effective in suppressing flaming combustion. However due to the electrical nature of battery packs, particularly the high voltages associated with large format battery packs, conductive suppression agents may not be a good choice. In addition, because of the potential for re-ignition due to cascading cell thermal runaway reactions, an ideal suppressing agent will stay suspended and prevent re-light of combustible mixture from cell hot surfaces. Suppressants shown to be effective include: inert gas / smothering of flames (fire behavior testing data indicates that smothering is effective in preventing flaming, but will not cool cells and prevent thermal runaway propagation), carbon dioxide (Exponent typically uses carbon dioxide extinguishers to suppress flaming of cells during testing – this will not cool cells and prevent thermal runaway propagation), water (anumber of sources have described the effectiveness of water to suppress flaming and cool cells), and Halon.
涉及锂离子电池的火灾是电解质的燃烧,这是一个烃/ airflame的结果。因此,许多的火焰灭火剂会有效抑制焰燃烧。然而,由于与大幅面电池相关的电池组,特别是高电压的电气性质包装,导电剂抑制可能不是一个很好的选择。另外,由于复燃,由于级联电池的热失控反应的可能性的,理想的灭火剂将保持悬浮,并防止电池热表面的可燃混合物的重轻。表明灭火剂是有效的包括:火焰的惰性气体/窒息(防火性能测试数据表明,窒息是有效预防炽盛,但不冷静细胞,防止热失控传播),二氧化碳(指数通常使用二氧化碳灭火器测试过程中抑制细胞的有焰燃烧 - 这不会冷却电池组,防止热失控传播),水和哈龙。
There is limited published data regarding the selection of suppressants for use on lithium-ion battery fires. The design of suppression systems in battery manufacturing facilities is generally considered proprietary information and is not publically available.
Testing data that is available has been published is related to very specific lithium-ion battery applications, primarily the suppression of fires in air transport: fires that might occur in a passenger cabin, where very limited numbers of cells could become involved and Halon extinguishers and water are available suppressants, and fires that might occur in aircraft cargo holds, where Halon is the available suppressant. Full scale fire suppression testing is necessary to evaluate specific storage configurations, quantities, arrangements and fire suppression system design criteria and overall effectiveness.
关于灭火剂对锂离子电池的公开的数据不多。在电池的生产设施灭火系统的设计通常被认为是专有信息,是不公开可用的。可用已公布的测试数据相关以非常具体的锂离子电池的应用中,主要是抑制火灾航空运输:可能发生在客舱,其中很火细胞的数量有限,有可能成为参与和哈龙灭火器和水可用抑制剂,和火灾可能发生在飞机货舱成立,其中哈龙是可用的抑制剂。满量程灭火测试是必要的特定存储配置,数量,安排和灭火系统的设计标准和总体评价效力。
Navy Sea Systems Command released an Advanced Change Notice for Lithium Battery Firefighting Procedures. In this document, the Navy recommends (based on limited testing), the use of “a narrow-angle fog of water or AFFF” to cool batteries, suppress “fireballs,” and reduce the likelihood of thermal runaway propagation.
The FAA studied suppression of lithium-ion batteries with water and Halon 1211, as these are typically available in hand extinguishers aboard commercial aircraft. As a first choice, the FAA recommends the use of water to suppress fires involving notebook computers, because water will both extinguish flames and suppress thermal runaway propagation. As a second choice, the FAA recommends using Halon 1211 to knock down flames, followed by deluge from available water sources (such as bottles of drinking water). Halon 1211 alone will not prevent re-ignitions of cells due to propagation of cell thermal runaway reactions. In FAA tests, application of ice did not sufficiently cool cells to prevent thermal runaway propagation.
海军海上系统司令部发布了一个先进的变更通知的锂电池消防手续。在该文件中,海军建议(基于有限的测试),使用的“窄角雾化水或水成膜泡沫“来冷却电池,抑制”火球“,并减少热失控传播的可能性。
美国联邦航空局研究了锂离子电池的抑制与水和哈龙1211因为这些是通常可在手灭火器船上商用飞机。作为第一选择,美国联邦航空局建议使用水抑制涉及笔记本电脑的火灾,因水将两者灭火和抑制热失控繁殖。作为第二选择,FAA建议使用1211打掉火焰,其次是从可用水源(如饮用水)。 1211本身并不能防止因电池组的复燃,电池热失控反应的传播。在FAA的测试中,冰的应用程序没有充分冷却电池,防止热失控的传播。
In 2010, the FAA reported on testing lithium iron phosphate and 8-Ah lithium cobalt oxide softpack polymer cells. Halon 1211 was able to successfully extinguish flames from these cells.
In addition, the iron phosphate cells did not continue to vent or re-ignite once the Halon 1211 was applied. However, Halon 1211 was not able to suppress re-ignition of the soft-pouch polymer cells (cobalt oxide chemistry).
2010年,美国联邦航空局报告的测试磷酸铁锂和8-Ah氧化钴softpack聚合物电池。1211能够成功从扑灭这些电池组的火焰。另外,磷酸铁电池没有继续发泄或复燃。然而,1211是不是能够抑制软袋聚合物单元的复燃(钴氧化物化学)。
Halon 1301 is the least toxic of the Halon fire suppressants and is considered to have superior fire extinguishing characteristics. In particular, it rapidly knocks down flaming combustion, has a penetrating vapor that can flow around baffles and obstructions, leaves no residue, is noncorrosive, requires small storage volumes, is non-conductive, and is colorless, which prevents the generation of false fire alarms by obscuration. Halon does not act by displacing oxygen, rather it acts by interfering with the chemistry of combustion, specifically by terminating chain branching reactions that occur in the gas phase in typical hydrocarbon/air flames.
The fact that Halon is effective in suppressing lithium-ion battery flames is another indication that these flames are substantially similar to typical hydrocarbon/air flames. Halon 1301 (bromotrifluoromethane) is a methane derivative. The bromine atom confers strong fire suppressant properties, while the fluorine atoms confer stability to the molecule and reduce its toxicity. Bromine atoms interfere with the free radical and chain branching reactions that are important in combustion.
哈龙1301是毒性最小的哈龙灭火剂,被认为是具有优越的灭火性能。特别是,它迅速击倒焰燃烧,具有穿透蒸气可绕流挡板和障碍物,不留残渣,无腐蚀性,要求小存储卷,是不导电的,并且是无色的,从而防止代通过遮拦消防警钟误鸣。哈龙不以行为置换氧气,而它作用通过用的化学干扰燃烧,特别是通过终止发生链支化反应在典型的烃/空气火焰气相。事实卤代烷是有效抑制锂离子电池的火焰是另一个指示这些火焰基本上类似于典型烃/空气火焰。哈龙1301(溴三氟甲烷)是甲烷衍生物。该溴原子表示授予强大的灭火剂特性,而氟原子赋予稳定性该分子并降低其毒性。溴原子干扰与游离激进和支链反应是燃烧重要。
Halon 1301 is generally considered very effective for electrical fires (Class C fires),flammable liquid and gas fires (Class B fires), and surface-burning flammable solid (such as thermoplastic) fires. However, Halon 1301 has minimal effectiveness on reactive metals, rapid oxidizers, and deep-seated Class A fires. Halon 1301 is minimally effective on deep-seated Class A fires because it works by interfering with the chemical reactions that create flames; it does notcool the fuel feeding the fire. Thus, while Halon 1301 can extinguish the flaming portion of a Class A fire, the glowing deep-seated portion of the fire can continue to smolder and spread at a reduced rate.
The strong effect of Halon addition can be seen upon examining the flammability limits of fuel/air/Halon mixtures, and comparing them with the flammability limits of fuel/air/inert diluent mixtures. When small quantities of Halon are added to a fuel/air mixture, they narrow the range in which that mixture is flammable. Halon is far more effective at narrowing the flammable range than an inert diluent. If sufficient Halon is added, the flammable range of a mixture, even at an elevated temperature, is eliminated and the mixture cannot be ignited. Note that production of Halon was banned by the Montreal Protocols, as this material contributes to the destruction of the ozone layer. Halon in use today is from recycled sources only, primarily for protection of aircraft.
哈龙1301通常被认为是电气火灾(C类火灾),易燃液体和气体火灾(B类火灾),以及表面燃烧可燃固体(如热塑性塑料)的火灾非常有效。然而,哈龙1301对活性金属,快速氧化剂,而深层次的A类火灾最少的有效性。哈龙1301是效果最差的深层次的A类火灾是因为它通过工作与创建火焰化学反应的干扰;因此,虽然哈龙1301可以扑灭燃烧一类部分火灾,大火烧红的深层次的部分可以继续在降低利率闷烧和蔓延。
卤代烷加成的强效可根据检查的燃料/空气/卤代烷混合物的可燃性限值,并将它们与可燃性比较可以看出燃料/空气/惰性稀释剂的混合物的限制。当哈龙的小批量加入到燃料/空气混合物,它们缩小范围,其中该混合物是易燃。哈龙是远远更有效地缩小可燃范围比惰性稀释剂。如果加入足够的卤代烷,则混合物的可燃范围,即使在高温下,被消除,并且该混合物不能点燃。需要注意的是生产哈龙的被蒙特利尔议定书禁止,因为这种材料有助于对臭氧层的破坏。目前使用的哈龙是唯一的再生资源,主要用于飞机的保护。
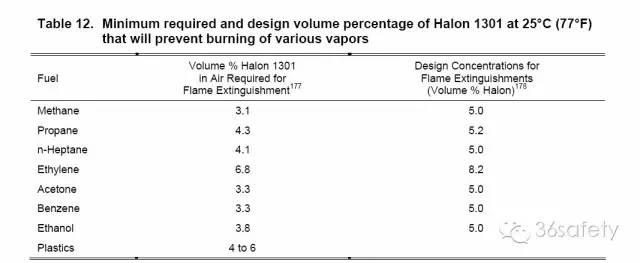
Table 12 shows the average percent by volume of agent in air required to extinguish a flame. It also shows the design concentrations for a total flooding system required to suppress flaming combustion. The design concentrations for flame extinguishment include an added safety factor over the required concentrations. These design recommendations are approximately 5% for most fuels.
表12示出了通过在扑灭火焰所需的空气剂的体积的平均百分比。这也显示了抑制焰燃烧需要全淹没系统设计的浓度。该设计浓度火焰熄灭包括在需要一个额外的安全系数浓度。这些设计建议约5%大多数燃料。
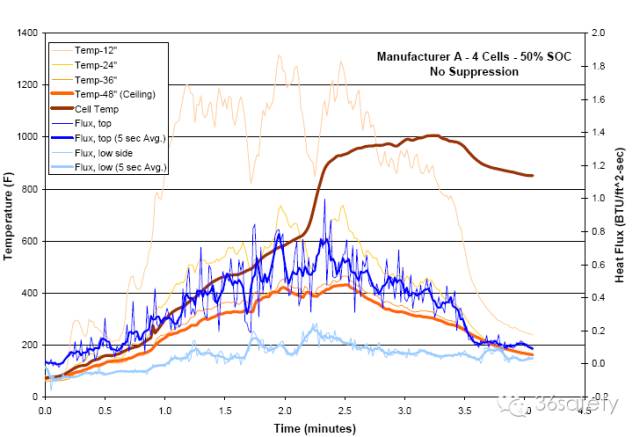
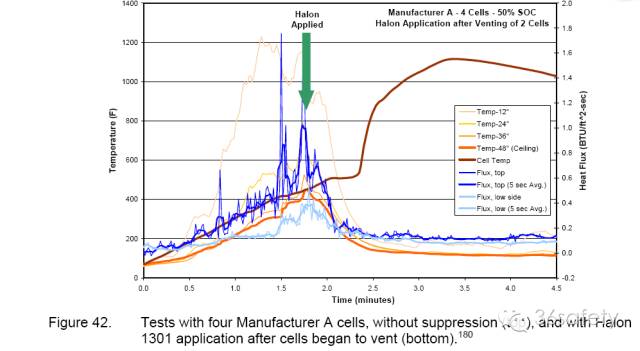
In 2004, Exponent conducted FAA-style testing of the effectiveness of Halon 1301 insuppressing lithium-ion cell and battery pack fires. A series of Halon 1301 suppression tests were conducted with bare 18650 cells and computer laptop battery packs. The bare cells were not electrically connected, but were taped together. Ignition was accomplished by igniting a pan of propanol below the cells. Halon 1301 was applied late in each test, once cells had begun tovent with burning jets. Within seconds of application, all flames were extinguished and no additional flaming was observed for the continuing duration of the test. When Halon 1301 was applied there was a precipitous drop in the chamber temperatures and heat flux measurements.
This was entirely consistent with flame suppression. Chamber temperatures and heat fluxes remained low for the duration of the testing. Note that Halon 1301 application did not cool the cells (Figure 42). Thermal runaway of individual cells and cell venting continued to occur after Halon 1301 was applied. Examination of all cells from Exponent’s Halon 1301 tests showed that they had vented. However, with Halon 1301 present, this process did not result in flaming combustion. Exponent concluded Halon 1301 is very effective in controlling burning lithiumion cells.
In 2006, the FAA conducted similar tests of Halon 1301 suppression on 18650 lithium-ion cells at 50 and 100% SOC.The FAA observed similar behavior to test results reported by Exponent.
2004年,指数进行了哈龙1301 insuppressing锂离子电池和电池组火灾的有效性FAA式测试。一系列的哈龙1301年抑制试验是用裸18650电池和笔记本电脑电池组进行。裸电池没有电连接,但被粘贴在一起。点火被点燃细胞下面丙醇的平移完成。哈龙1301在每个测试后期应用,一旦细胞已经开始用燃烧的飞机发泄。在应用秒钟,所有明火被扑灭,并观察到了测试的持续时间不断没有额外的炽盛。当哈龙1301应用于有在室内的温度和热通量测量值的急剧下降。
这与火焰的抑制是完全一致的。腔室的温度和热通量对于测试的持续时间仍然很低。需要注意的是哈龙1301的应用程序没有冷却细胞(图42)。单个细胞和细胞排气热失控继续应用于哈龙1301后出现。从指数的哈龙1301的测试所有细胞的检查表明,他们已经排出。然而,卤化烃1301目前,这种方法并没有导致。指数总结哈龙1301在控制锂离子燃烧细胞非常有效。
2006年,美国联邦航空局50 18650上的锂离子电池进行哈龙1301的抑制类似的测试,100%SOC. 联邦航空局观察到类似的行为来测试通过指数报告的结果。
本文内容摘自:
Lithium-Ion Batteries Hazard and Use Assessment
锂离子电池危险以及使用评估(由美国消防保护基金会赞助)
注:Google翻译有很多不当之处,限于时间原因,未能校正。请读者自行
校正。





